
Now, researchers all over the world are seeking mRNA for their subsequent product, particularly in the discipline of cancer research. For this to work, dependable strategies for mRNA purification need to be established. To assist mRNA research and therapeutic applications, Seattle Genova offers custom mRNA synthesis and its purification with rapid completion time, a huge variety of deliverables, and high quality. Experts in Seattle Genova make sure to provide customers with purified high quality product everytime.
Mechanism
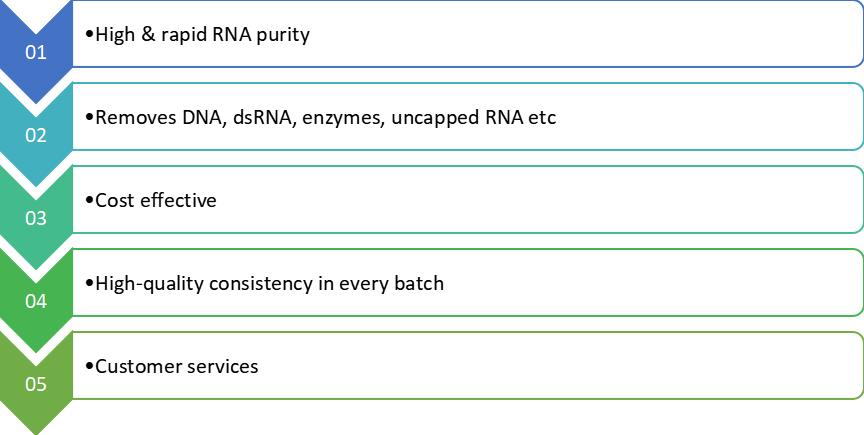
Following the in vitro transcription step, mRNA is purified from the impurities and substances used in the previous steps which include endotoxins, immunogenic double-stranded RNA (dsRNA), residual DNA template, RNA polymerase, and elemental impurities. Seattle Genova offers technologies for mRNA purification:
Tangential Flow Filtration (TFF)
Chromatography
Normal Flow Filtration
Tangential Flow Filtration
TFF permits efficient separation of mRNA from smaller impurities that aren't retained through the membrane; molecular weight cut-offs starting from 30 to 300 kDa may be used primarily based on the scale of the mRNA. With TFF it is feasible to purify, concentrate and diafilter the product in the same unit operation. At this stage, the mRNA will need to be in the suitable buffer, both for enzymatic capping or chromatography. In Seattle Genova, experts make sure to avoid that small DNA fragments cannot hybridize to the mRNA, producing extra impurities.
Chromatography
A variety of chromatography techniques may be used as an alternative to TFF and include:
Reverse-phase ion pair
Anion exchange
Affinity chromatography.
Chromatography offers an efficient approach for DNA template elimination and removes the threat of hybridization that could arise all through.
Normal Flow Filtration
Following the chromatography step(s), a final concentration and diafiltration is carried out to maximize product purity and transfer the mRNA into the suitable buffer for storage. At this stage, mRNA may be further purified, concentrated and diafiltered in the same unit operation. A sterile filtration step can be finished following this TFF step.
Highlights
Deliverables
Purified mRNA is supplied as a dry powder, completely lyophilized and shipped with dry ice.
A complete work report.
Thank you for your interest in our mRNA Purification Services services. Please complete the form below and we will contact you shortly.
Fields indicated by an * are required.